Well-Established Procedure with 5-Year Data in USA and Asia
FDA Approved 5-Year Data*
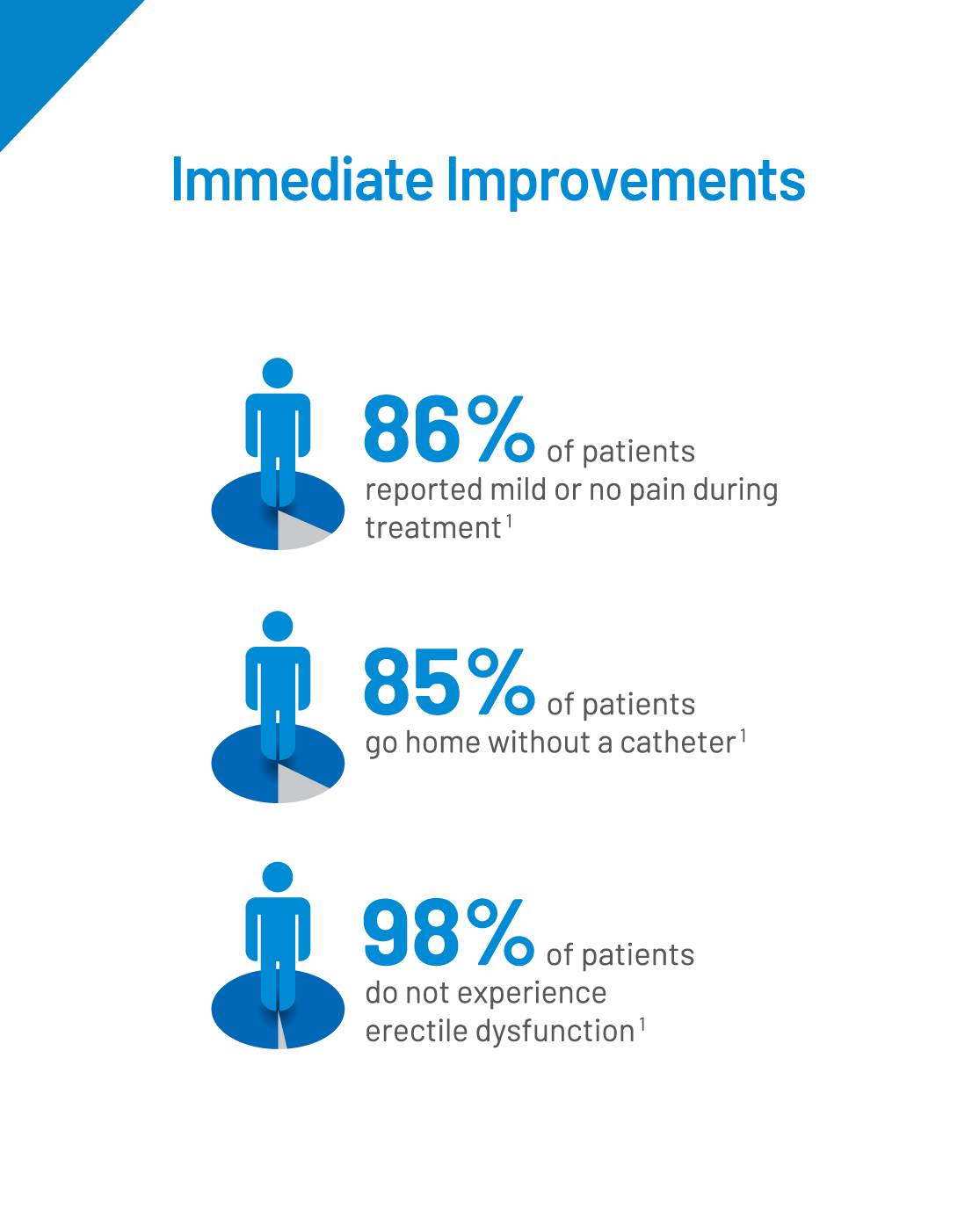
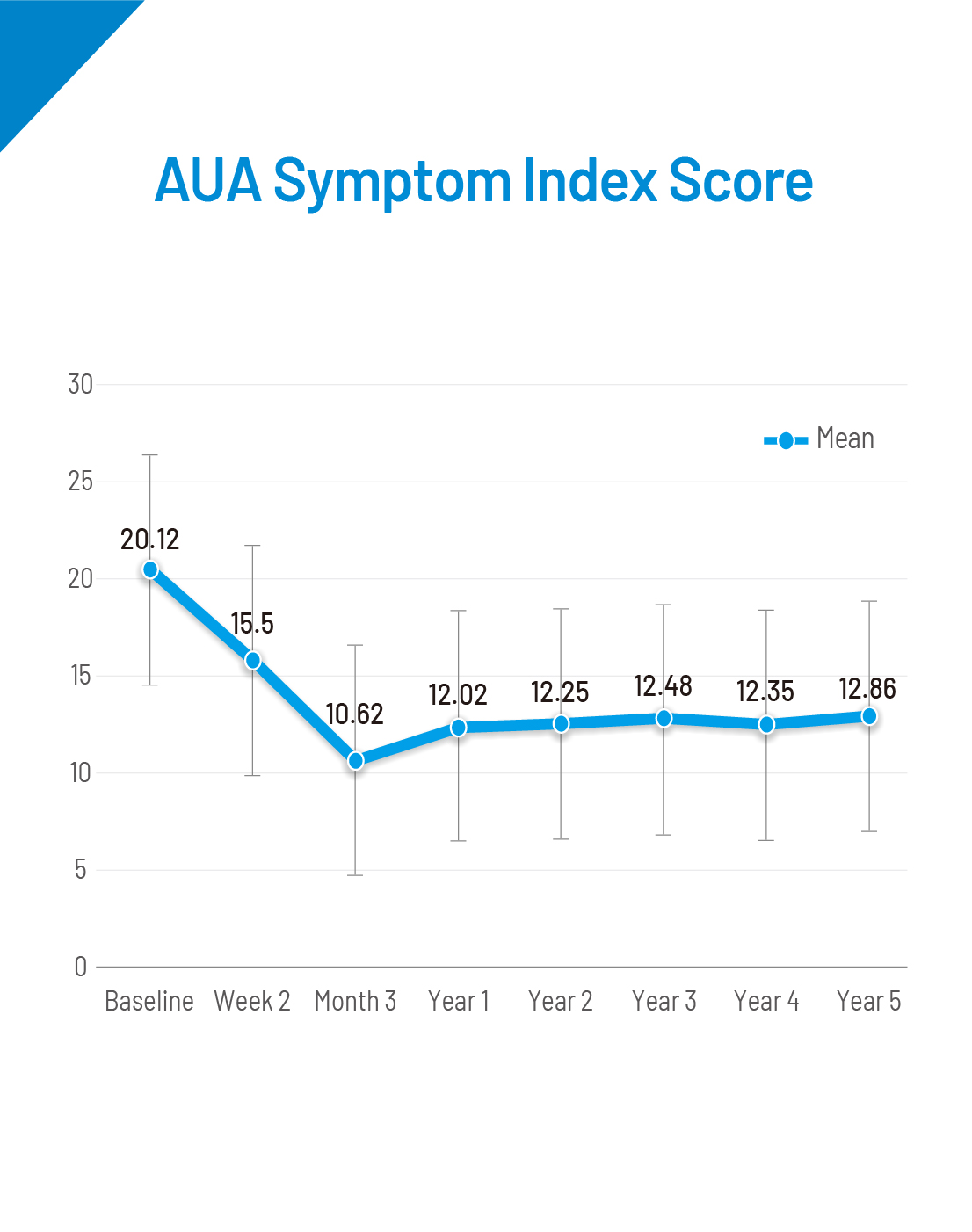
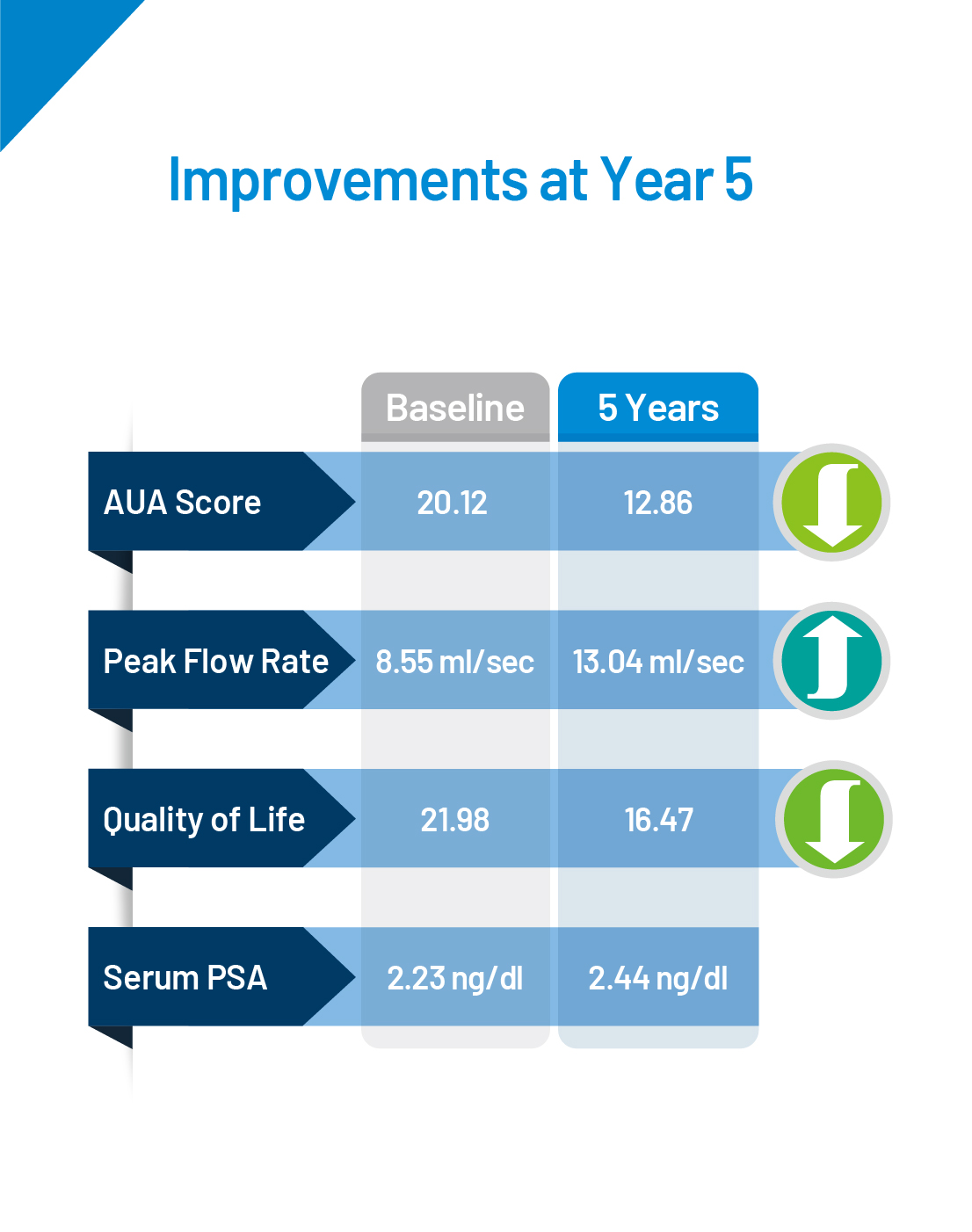
A total of 225 subjects were enrolled for the Prolieve post-market study. The study was conducted to evaluate the long-term effectiveness of the Prolieve System. All parameters were studied in comparison of change of baseline at Week 2, Month 3, Year 1, Year 2, Year 3, Year 4 and Year 5 among all subjects. Inclusion criteria: Diagnosed with BPH, peak urine ow rate <12 mL/sec on voided volume of >125mL, AUA symptom Score value >9, prostate weigh of 20-80g, prostatic urethra length of 1.2-5.5cm.
Asia Follow-Up Data
Following the FDA approved protocol of the 10 Year Post Market Study with 5-Year Follow Up, a similar study was conducted in Asia to evaluate the long-term effectiveness of patients treated by Prolieve. The results indicated that the immediate and long-term symptomatic relief of American and Asian patients are consistent. Study results were presented at various urology conventions as follows.
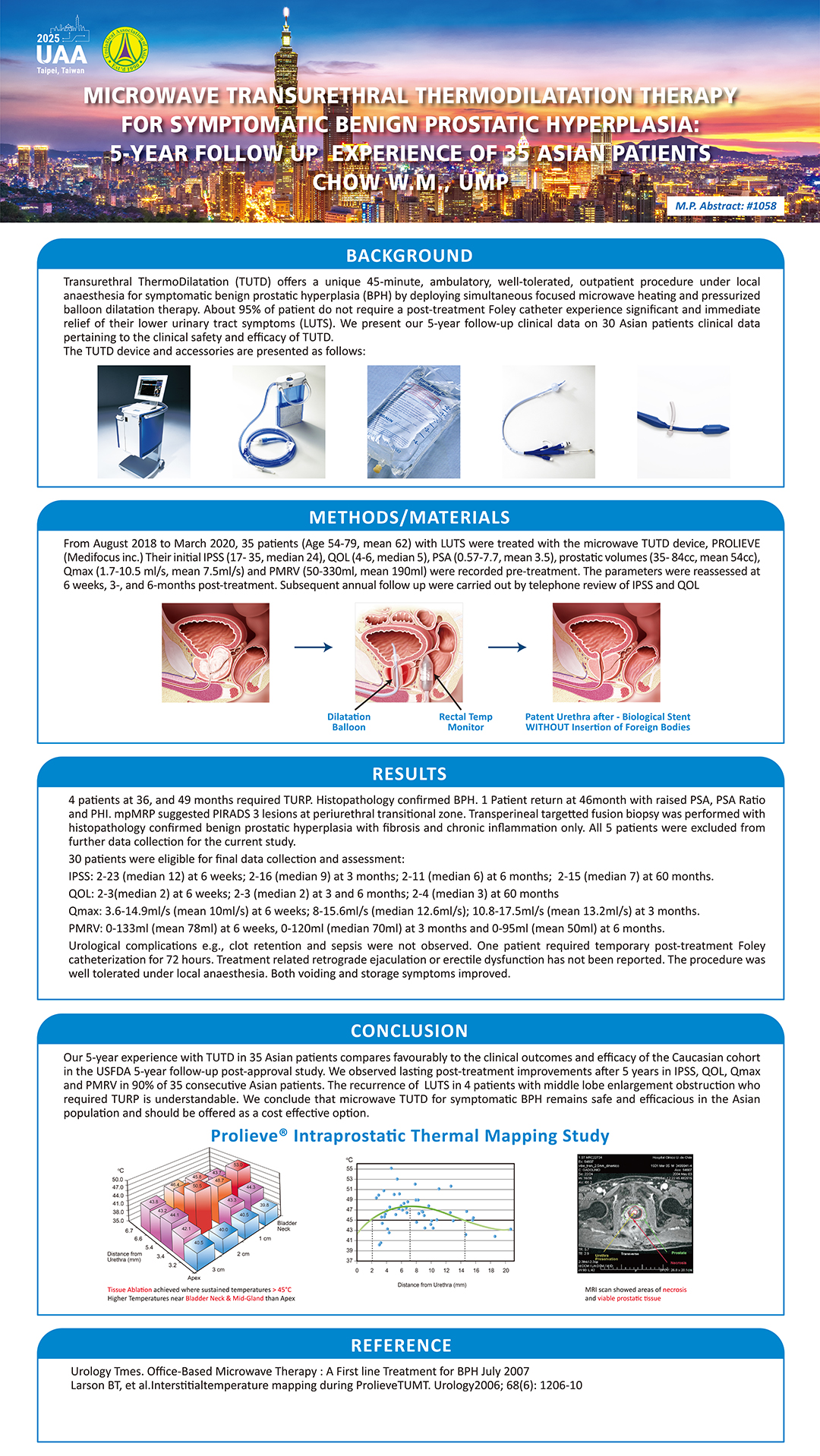
- UAA 2025: 5-YEAR FOLLOW UP OF ASIAN PATIENTS TREATED BY PROLIEVE

- WCET 2024: 4-YEAR FOLLOW UP 30 PATIENTS
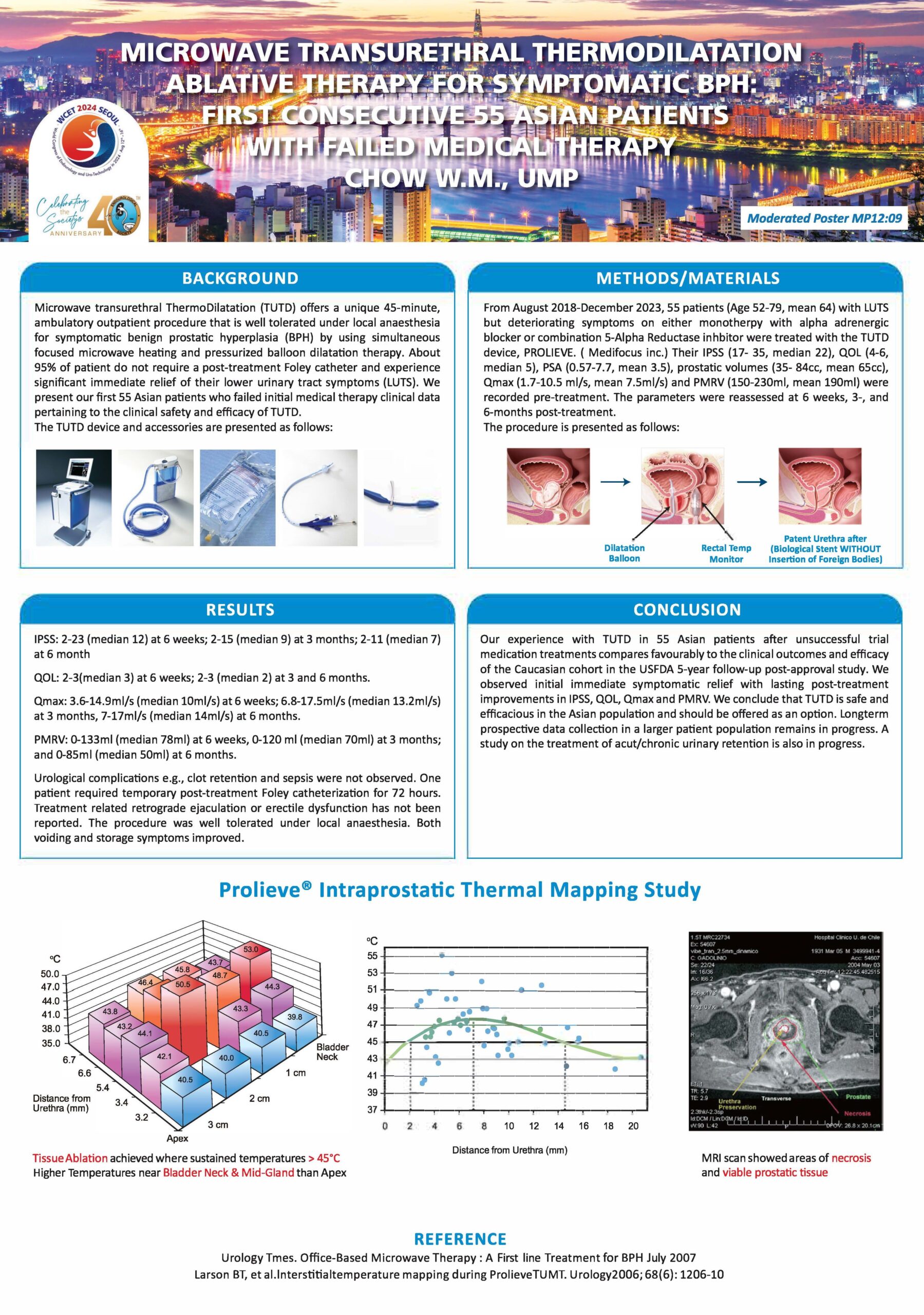
- WCET 2024: RESULTS OF FIRST CONSECUTIVE 55 PATIENTS WITH FAILED MEDICAL THERARY
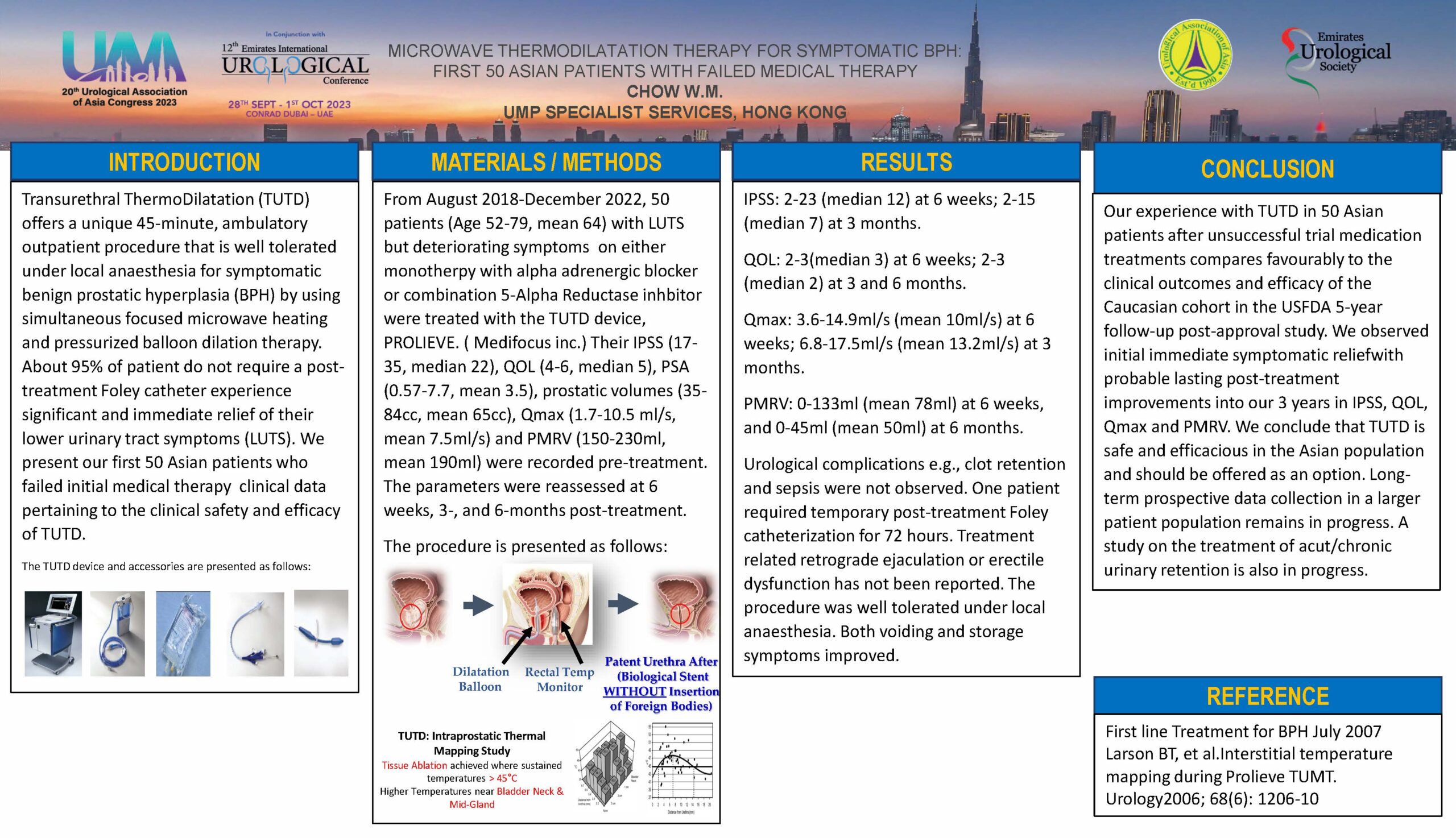
- UAA 2023: RESULTS OF FIRST 50 ASIAN PATIENTS WITH FAILED MEDICAL THERAPY
*Bidargaddi, V & Mon, J, ‘Post-Marketing Study Using Prolieve for the Treatment of Benign Prostatic Hyperplasia (BPH), 120-Month Post-Approval Study Annual Status Report, Unpublished Report, February 19, 2004, Columbia, United States.

